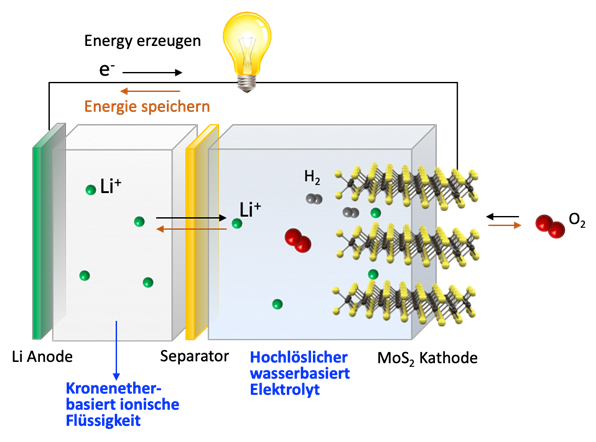
Metal-air batteries make it possible to store large amounts of electricity in a small space. Zinc-air batteries, for example, are used in hearing aids because of their high energy density. However, such metal-air batteries cannot yet be recharged. Unlike the discharging of the lithium-ion batteries widely used in mobile phones and other devices, that of metal-air batteries is difficult to reverse. A particular problem arises when the metal used is lithium, as it needs to be protected against exposure to oxygen. However, the challenge is worth taking on, as this type of battery features the best energy storage capacity.
In this project, the researchers have succeeded in developing new crown ether-based ionic liquids as electrolyte additives that allow for reversible charging and discharging of a lithium-air battery. Crown ethers are ring-shaped molecules at the centre of which lithium ions can be transported. Being ionic liquids, they are flame-retardant and safe to handle, unlike conventional electrolytes. In addition, the researchers have constructed novel molybdenum disulphide membranes that can be placed on the cathode side of the battery for selective oxygen supply. These membranes are stable even at high pH values and are not subject to clogging during the charging and discharging process. Using these components, the scientists have built a working prototype in the laboratory that can be successfully recharged.
These findings could lead to the development of a new generation of safe, rechargeable lithium-air and lithium-water batteries that provide a higher energy density and are suitable as self-sufficient storage systems.