Presently, a significant proportion of greenhouse gas emissions are caused by road traffic. Consequently, in order to achieve the climate targets, cars and trucks must forgo fossil fuels and switch to electric power. The electricity required can be provided either by batteries or by on-board hydrogen which is converted into electricity in so-called fuel cells. The only waste product is pure water, i.e. no carbon dioxide, nitrogen oxides or particulate matter. The advantage of fuel cells in buses, trucks and cars, as opposed to batteries, is that vehicles can be refuelled with hydrogen very quickly, as is currently the case with petrol. This eliminates long waiting periods while a battery is being recharged. The high energy density of hydrogen enables vehicles to achieve long ranges. A new value chain in the field of hydrogen and fuel cells, to which researchers from the Zurich University of Applied Sciences ZHAW are also contributing, is currently emerging worldwide.
Fuel Cells for Sustainable Mobility
Electric cars can draw their electricity from batteries. Hydrogen, too, is a source of energy. In fuel cells, it can be converted into electricity without CO2 emissions. Researchers have optimized these fuel cells and are now bringing the outcome to market on a website.
At a glance
- Hydrogen and fuel cells can serve to store electricity for later purposes. Swiss researchers are now improving the efficiency of this technology.
- Improving the transport of hydrogen and oxygen through porous gas diffusion layers can reduce losses within the cells.
- Researchers at the PSI and the ZHAW have therefore studied the processes in the pores on a microscopically small scale. Using the resulting data, they have developed mathematical models they are now marketing for commercial purposes.
Micropores within the cell
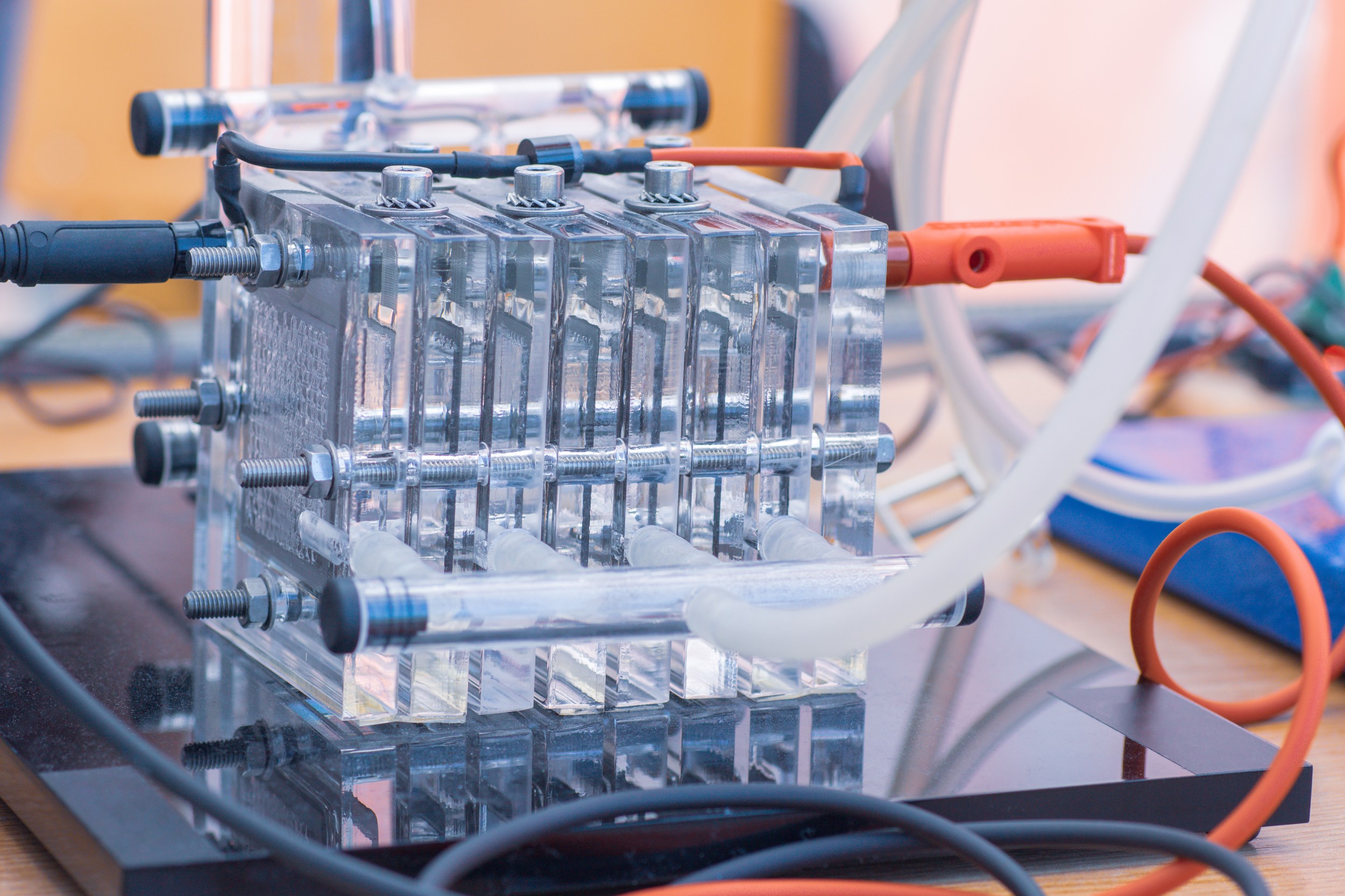
More specifically, the researchers have studied so-called polymer electrolyte fuel cells (PEFC). In these fuel cells, the reaction components, oxygen and hydrogen, must diffuse through a porous gas diffusion layer. However, at high specific performance, measured by weight, considerable electrical voltage is lost during transport of the reaction components. In order to reveal the conditions under which this happens, the researchers screened the gas diffusion layers of fuel cells using X-ray tomography, an imaging process that enables the visualization of minute structures. This allowed them to establish a connection between transportability and properties such as pore distribution, the resistance of narrow pores to transport processes, and non-circular pores. In doing so, they were able to quantify the transportability of different gas diffusion layers. The scientists then compared the X-ray measurements with computer simulations and developed mathematical models that allow fuel cell developers to build more efficient devices. One possible application is the development of new materials with optimized pore designs for gas diffusion layers. The researchers are now in the process of setting up a web platform to market the computer models, so that, in the future, all can benefit from this research work.
How does a fuel cell work?
Fuel cells can be used to technically exploit the energy stored in hydrogen. In these cells, hydrogen and oxygen are brought together under controlled conditions. A chemical reaction then produces electricity and, as a waste product, water.
The electricity is generated because hydrogen and oxygen react with each other (the so-called detonating gas reaction is well known). Hydrogen and oxygen are supplied to the fuel cell separately, allowing the chemical reaction to occur under controlled conditions rather than explosively. Gas diffusion layers are used to achieve an even distribution of the gases on the electrochemically active surfaces. In devices known as gas diffusion electrodes, hydrogen is split into an H+ and a negatively charged electron on the anode side. On the cathode side, oxygen is also reduced. Only positively charged particles can pass through a proton-conducting membrane between the two cell sides. The negatively charged electrons generated from the hydrogen must find another way to meet the oxygen on the other side of the barrier and then react to form water. This alternative route is an electrical conductor, in which the flow of electrons generates an electric current. The resulting electricity can then be used to power various devices.
Products of this project
Contact and Team
Prof. Jürgen Schumacher
ZHAW School of Engineering
Forschungsschwerpunkt Electrochemical Cells & Energy Systems
Wildbachstrasse 21
8400 Winterthur
+41 58 934 69 89
juergen.schumacher@zhaw.ch
Felix Büchi
Jürgen Schumacher
Project direction
All information provided on these pages corresponds to the status of knowledge as of 12.06.2019.

